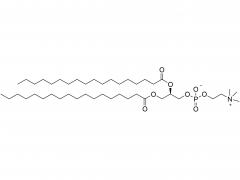
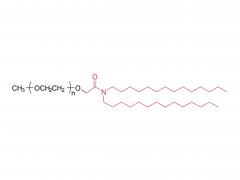
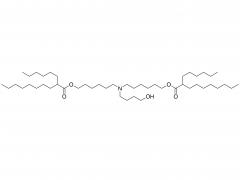
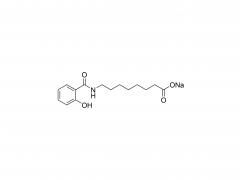
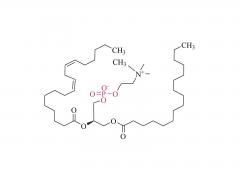
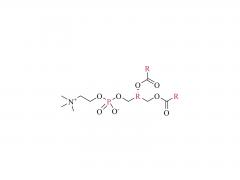
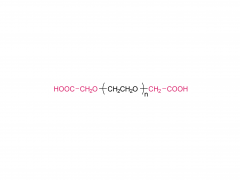
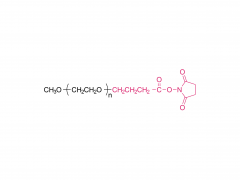
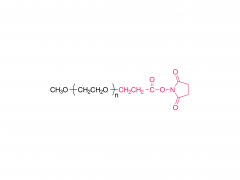
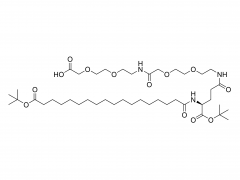
Polyethylene glycol (PEG) is a glycol polymer synthesized by the polymerization of ethylene oxide, with a relative molecular weight (Mr) mainly between 200 and 40,000. With the increase of relative molecular weight, the physical appearance and properties of polyethylene glycol gradually change: liquid in the range of relative molecular weight of 200 to 600, and gradually become semi-solid above 600. Polyethylene glycol not only has good water solubility, but also soluble in organic solvents such as benzene, acetonitrile, ethanol.
Its physicochemical properties include:
unique amphiphilicity: this structure makes polyethylene glycol soluble in both organic solvents and water.
Non-immunogenicity: even with a relative molecular weight as high as 5.9×106 Da, its immunogenicity is very low. At present, the production of anti-polyethylene glycol antibodies has not been observed when using polyethylene glycol modified proteins in clinical treatment.
Non-toxicity: studies have shown that polyethylene glycol with a relative molecular weight greater than 1000 is non-toxic and has been used in a variety of foods, cosmetics and drugs.
Biodegradable: polyethylene glycol can be removed directly from the body without any structural changes. Polyethylene glycol with a molecular weight less than 20000 is metabolized by the kidney, while larger molecular weights can be metabolized by the digestive system (slowly excreted through urine or feces).
It is worth noting that polyethylene glycol is one of the very few synthetic polymers approved by the US Food and Drug Administration (FDA) for use in injectable drugs.