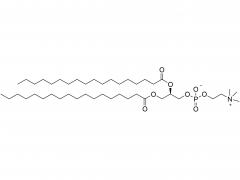
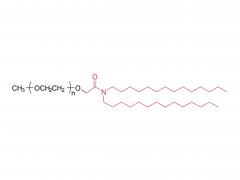
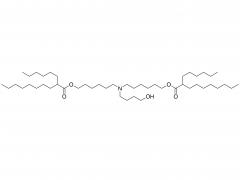
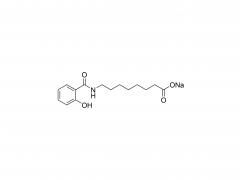
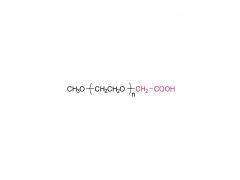
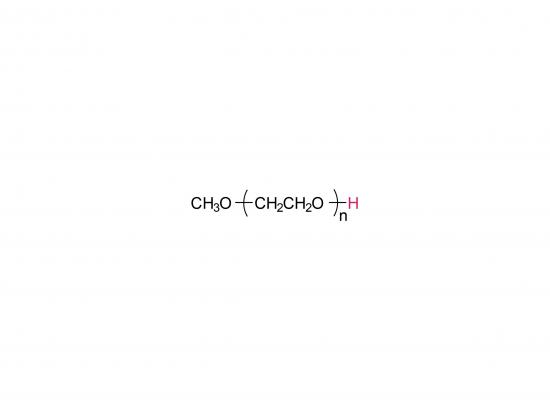Methoxypoly(ethylene glycol)
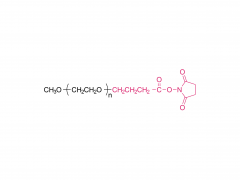
SINOPEG manufactures high-purity methoxy-PEG-OH characterized by absence of contaminating impurities and narrow molecular weight distributions. These highly pure methoxy-PEG-OH have so far been used for various commerical PEGylated drugs, such as PEG-Interferon and PEG-GCSF.
Our methoxy-PEG-OH has the davantage of extremely low diol contents relative to those of competitors' products. Our methoxy-PEG-OH contains remarkably low levels of diol as impurities, besides providing a narrower distribution of molecular weights in comparison with our competitors' products. Employment of our methoxy-PEG-OH as the starting materials yields higher purity and higher activity of Actives PEGs. We manufacture highly pure methoxy-PEGs with pure methoxy-PEGs with molecular weights form 5KDa to 80KDa.
Our products are industrially recognized, industry leading benchmark products for key performance attributes: high LOT-to-LOT consistency, accurate molecular weight control, impurity profile (low impurities), strong analytical development work for each product to support drug dossier preparation.
SINOPEG is serving pharmaceutical and medical device companies around the globe, with product presence in various pharmaceutical/device development pipeline (pre-clinical, clinical, and post authorization large scale supply). Our facility is ISO9001 and ISO13485 certified, and is operating according to ICH Q7A guidelines to produce products for pharmaceutical companies.
Please contact us at sales@sinopeg.com for PEG derivatives. Our online catalog or inventory may not listed or have all molecular weights and functional groups, which may be available by custom synthesis. Please contact us at sales@sinopeg.com for quotation and availability.
License is required from license holder.