Icodec insulin is a long-acting insulin analog that is injected once a week for the treatment of diabetes. On March 21, the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion recommending approval of Novo Nordisk Awiqli, a weekly basal insulin formulation of icodec insulin, for the treatment of diabetes in adults. Novo Nordisk (NVO.US) is expected to receive final marketing authorization from the European Commission in approximately two months.
Awiqli is a basal insulin that is administered once weekly by subcutaneous injection. In six confirmatory randomized clinical studies, the efficacy of once-weekly injections of icodec insulin was compared to once-daily injections of basal insulin. The results showed that icodec insulin had a similar duration of hypoglycemia (blood glucose values <3.9 mmol/L) in terms of lowering HbA1c levels as once-daily injections of basal insulin at both the treatment transition and treatment steady state periods. This demonstrates that Icodec insulin combines efficacy and safety and achieves an extraordinarily long half-life. It is expected that Icodec insulin will play an important role in the field of diabetes.
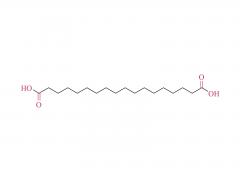
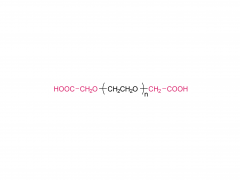
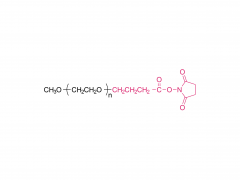
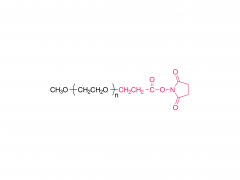
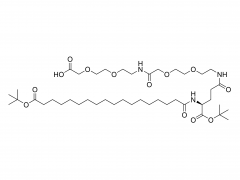
Unlock the innovation that will help you stand out in the field of diabetes treatment! Sanobango offers a wide range of fatty acid-modified side chains to support long-acting drug research. In particular, we recommend icodec insulin side chains, which are available in quantities in stock, to help you make a successful breakthrough! Welcome to contact us for a win-win future!