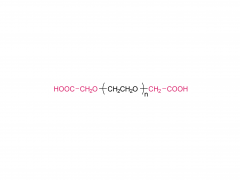
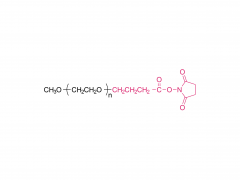
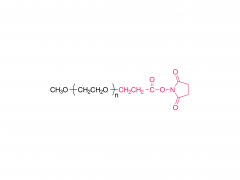
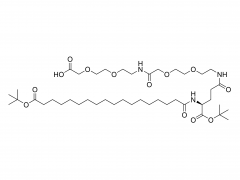
Tetra-PEG hydrogels based on the ammonolysis reaction between tetra-armed poly(ethylene glycol) amine (Tetra-PEG-NH2) and Tetra-PEG-SAE offer massive advantages as sealants. They are entirely synthetic without the misgivings of being inhibited by anticoagulation agents and transferring disease. Their cost is low due to their easily preservable components with high accessibility. Because of the intrinsic properties of this ammonolysis reaction, the resulting hydrogels can gel fast just by injection and adhere to the tissues tightly throughchemical bonds. Another remarkable advantage for Tetra-PEG hydrogels is that they are mechanically tough, and the sealants are favored to be mechanically tough to keep stable in case of dynamic movement of the tissues and the use of assistant pressure which is a key adjunctive step in achieving hemostasis. However, two hurdles are preventing extending their applications in vivo. The first one is that just as commercialized sealants, none of the reported Tetra-PEG hydrogels could be controllably removed without mechanical debridement, which is extremely dangerous because of their high mechanical strength. Besides, they possess long degradation time, which will lead to severe foreign body reactions, tissue adhesion, disturbed tissue healing, and obstruction of the circulatory system, when used in vivo. Here, to overcome the limitations of the existing ammonolysis based Tetra-PEG hydrogels, we construct an optimized one (SS) with fast degradable and controllably dissolvable properties via Tetra-PEG-NH2 and tetra-armed poly(ethylene glycol) succinimidyl succinate (Tetra-PEG-SS) . The resulting SS exhibits biocompatibility superior to the reported degradable Tetra-PEG hydrogel (SG) based on Tetra-PEG-NH2 and tetra-armed poly(ethylene glycol) succinimidyl glutarate (Tetra-PEG-SG) . More importantly, in contrast to the disappointing results of SG that leads to serious adverse effects in in vivo hemostasis due to the long retention, SS causes almost no noticeable side effects with outstanding hemostasis efficacy even under the anticoagulated situations. This hydrogel is a promising candidate for the next-generation in vivo sealants in the aged society.
View More