Block copolymers constitute an imperative class of multiphase systems that have emerged as important materials in the past several years and aroused a worldwide interest to physicists, chemists, biologists, and chemical engineers, and has been widely used in medicine, construction and chemical industry. Block copolymers are composed of chemically different homopolymers covalently connect at one end, each polymer of block copolymer can be called a block. One of the most interesting properties of block copolymers is their ability to self assemble into ordered microdomain structures.
The amphiphilic block copolymers are usually linear (diblock, triblock, and multiblock), cyclic, and miktoarm. The chemical nature of the constituting blocks, their composition, and total molecular weight exhibits varied solution behavior and excellent properties.
The current advances of block copolymers are strongly related with the development of new proficient polymerization techniques. Block copolymers can usually be obtain by (a) sequential controlled or living polymerization, (b) simple coupling reaction, (c) using a dual initiator of 2 different initiating fragments, and (d) the macro-initiators including functionality switching. It is notable to point out that sometimes copolymers with a certain desired structure are impossible to be synthesized only by a single polymerization technique. In such case, the combination of different synthetic techniques and function of switching groups are feasible strategies.

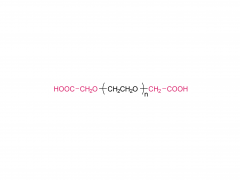
PEG is a hydrophilic, nonionic polyether that has been shown to exhibit excellent biocompatibility, and approved by the FDA for internal consumption, PEG is a neutral polymer with hydroxyl end groups that are weak hydrogen bond acids, and weakly basic ether linkages in the backbone. PEG molecules can be added to drug delivery vehicles via a number of different routes including covalent bonding, blending during preparation, or surface adsorption. It can be modified at either end group for attachment to other molecules or polymers. PEG, which was originally introduced in the pharmaceutical field with the aim of extending the half-life of proteins in blood, reducing their immunogenicity, and protecting them from proteolytic degradation, is widely chosen for developing block copolymeric because of its hydrophilicity, linearity, chain flexibility, lack of charge, and availability in a wide range of MWs with narrow MW distribution.
A biodegradable polymer is considered as “green” because it does not accumulate in the body and does not harm the environment, showing the attractive application in the field of biomedical or drug delivery owing to its ability to be easily metabolized and excreted from the body. Biodegradable materials are not restricted to the site-specific targeting of drug, peptide, and protein but also are increasingly important in medical devices and wound dressing. To meet these requirements, biodegradable block copolymers have been investigated as promising biomaterials because of their ability to modify their amphiphilic behavior, physical, and mechanical properties by altering the ratio of consisting blocks or adding new blocks of desired properties.
Block copolymers exist as unimers below their critical micelle concentration (CMC) and form core-shell-type macromolecular assemblies as polymeric micelles above CMC. These resultant polymeric micelles formed are in equilibrium with their respective unimers. The micellization of such polymers in water resembles the self-assembly of surfactants. Polymeric micelles are amid the extensively studied delivery platforms where the outer shell minimizes opsonization and the inner core deals with drug solubilization, a prerequisite to drug transport. Poor aqueous solubility of hydrophobic drugs limits the success rate during highthroughput screening. This poses a challenge for the formulation of scientists, particularly taking into account the growing number of approved molecules with high molecular weight, melting point, and lipophilicity. The traditional approach of solubilization in lipid-based formulations suffers from rapid drug precipitation in vivo, prior to absorption. Contrarily, micelles exhibit superior thermodynamic stability and biocompatibility.

Recently, research interest has been raised in the application of block copolymer micelles as the nanocarrier system in the field of drug delivery because of the hydrophobic drug-loading capacity of the inner core as well as the unique in vivo disposition characteristics. These block copolymer micelles offer many advantages as effective drug delivery systems including (1) facile preparation, (2) colloidal stability with low critical micelle concentration (CMC), (3) tunable sizes with narrow size distribution, (4) the ability to protect drugs from possible deactivation and preserve their activities during circulation, and intracellular trafficking, (5) improved pharmacokinetics, and (6) high physical loading efficiency of drugs without chemical modification.
Several biodegradable plastic polymers, such as polyethylene glycol (PEG), polylactic acid (PLA), polycaprolactone (PCL) and their block copolymers, were developed for biomedical applications. As a research focus in recent years, polyethylene glycol-polylactic acid (PEG-PLA) block copolymer and its end-group derivative nanoparticles can enhance the drug loading of hydrophobic drugs, reduce the burst effect, avoid being engulfed by phagocytes, increase the circulation time of drugs in blood, and improve bioavailability.
Conventional chemotherapy lacks specific targetability to the tumor as a result of which, several cases of drug exposure to the healthy cells and drug resistance are emerging. Also, the low aqueous solubility of anticancer drugs is a restraining factor in drug design. Block copolymers can circumvent this problem due to their self-assembly characteristics and high drug loading efficacy. Tunable physicochemical properties and further functionalization serve very promising excipients for the formulation development. Block copolymeric micelles do undergo passive distribution and retention within the targeted site via enhanced permeability and retention effect. It facilitates the transport and localization of macromolecules or particulates with minimal exposure and damage to the healthy cells. Therefore these nanoaggregates have gained widespread attention and a huge amount of optimism in drug delivery and targeted therapies as they are thermodynamically stable, biocompatible, and less toxic. Still, the drug distribution is heterogeneous due to the nonuniformity of vasculature. However, this distribution can be rendered more specific through chemical modification of the polymer backbone that enables a selective and high-affinity interaction of the functionalized carrier with the target cells.
Block copolymeric micelles entrap the lipophilic drug in its hydrophobic microenvironment core and enhance its solubility and bioavailability. Their self-assembly is driven by the difference in water solubility between hydrophilic and hydrophobic blocks. As the temperature increases, the association number enhances progressively, thereby inferring an anisotropic micellar growth or structural transition. Studies have also shown the mixed micellar approach that typically combines two block copolymers with varying hydrophilic-lipophilic balance values in order to enhance the drug encapsulation and stabilization.
In summary, block copolymer-based drug delivery systems hold great potential for improving the efficiency of therapeutic molecules and minimizing their harmful side effects. As well as their self-assembly behaviors, which are critically important in the fields of medicine, life, biotechnology, and environment, and will provide unexpected applications.
References
1. Kuperkar, K., Tiwari, S., and Bahadur, P. Self-Assembled Block Copolymer Nanoaggregates for Drug Delivery Applications. Applications of Polymers in Drug Delivery, 2021, 423–447. 10.1016/B978-0-12-819659-5.00015-X
2. Raval, N., Kalyane, D., Maheshwari, R., and Tekade, R. K. Copolymers and Block Copolymers in Drug Delivery and Therapy. Basic Fundamentals of Drug Delivery, 2019, 173–201. 10.1016/B978-0-12-817909-3.00005-4
3. Giram, P. S., Wang, J. T.-W., Walters, A. A., Rade, P. P., Akhtar, M., Han, S., Al-Jamal, K. T. Green synthesis of methoxy-poly(ethylene glycol)-block-poly(l-lactide-co-glycolide) copolymer using zinc proline as a biocompatible initiator for irinotecan delivery to colon cancer in vivo. Biomaterials Science. 2020. 10.1039/d0bm01421d